Uterine care that’s just right
Minimally invasive, uterus preserving, no hormones.
Meet Minerva in the middle. At the intersection of technology and gynecology. Experience modern care that preserves your uterus, while treating AUB.
“This feels just right”
The devices matter
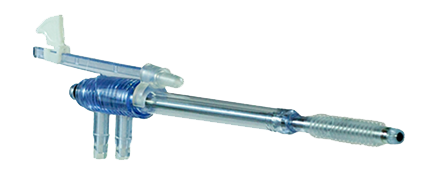
Minerva's minimally invasive devices help physicians target the causes of AUB, rather than medicate the symptoms or remove the organ altogether. Each treatment is minimally invasive and, in many cases, is performed in the physician’s office.
Minerva's treatments are minimally invasive, modern and safe.
Engineered to elevate the experience for you and your physician. Designed to improve upon all those devices that came before.
Each treatment is hormone-free and preserves the uterus.
Treatments for the Endometrium
The most effective endometrial ablation treatment in the history of clinical trials conducted for FDA approval.1
Endometrial ablation is a one-time treatment that is effective at reducing or eliminating bleeding and clots due to AUB-E. The treatment involves a minimally invasive method of removing the uterine lining. This is the layer that sheds during your period.
Designed by the same team that developed NovaSure in the 1990s, Minerva ES was judged to be statistically significantly more effective1, in 2015, when the FDA compared Minerva ES to a group of product treatments that included NovaSure® and 4 other previously FDA approved products.
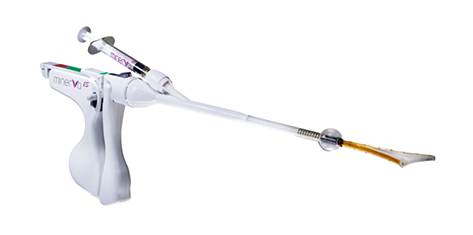
The soft silicone tip of Minerva ES changes the entire experience.
PlasmaSense Technology™
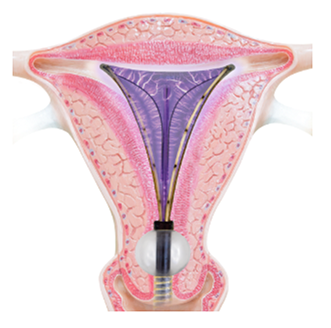
Minerva ES uses heated plasma energy that is fully contained within the silicon tip, rather than the NovaSure electrified metalized mesh that is in direct contact with the uterus.
NovaSure requires the removal of moisture from the uterine cavity due to the exposed, electrified metallized mesh.
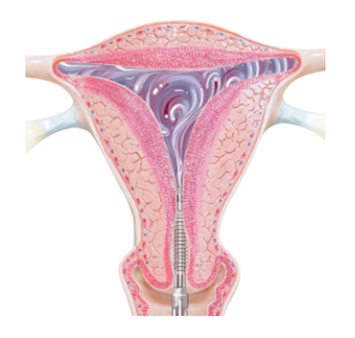
The soft silicone membrane on Minerva ES allows for the moisture to remain so it can flow throughout the uterine cavity, reaching areas that do not come in contact with the membrane, for complete coverage.
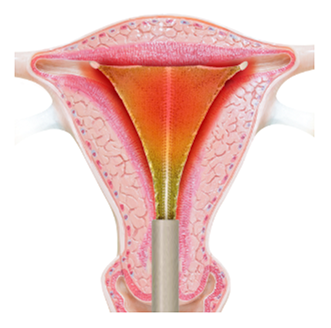
The heat and remaining moisture remove the top layer of the endometrium, which is the lining of the uterine cavity. This takes just 120 seconds.
At the end of two minutes, the device is closed and your doctor gently removes it from the uterus. The silicone covering ensures removal without sticking.
Thank you, Engineering.
PlasmaSense technology eliminates the need for uterine tissue to come in direct contact with the Minerva ES device. The NovaSure device requires direct tissue contact, which is created by using a vacuum to pull the uterine walls directly onto the device. This vacuum also removes the moisture that builds up in the uterus during the ablation cycle in order to ensure continued direct contact between the uterine wall and the device. With Minerva ES, the moisture remains and helps ensure all uterine tissue is thermally ablated from either the moisture itself or from being in contact with the silicone array.
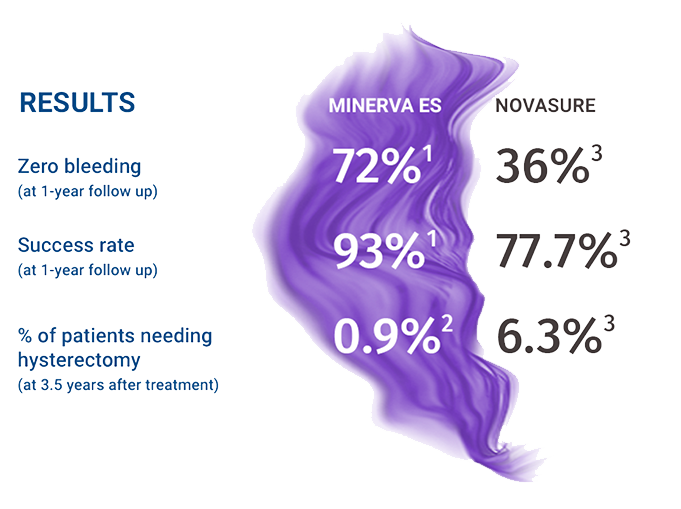
Minerva ES VS NovaSure
Clinical data comparison
(from separate clinical trials for FDA approval)
For the unique uterus
Genesys HTA®
Heated saline endometrial ablation under direct visualization
Genesys HTA is an alternative endometrial ablation system to Minerva ES. It is designed to help ensure that a woman with an atypically shaped uterus has access to an ablation.
Instead of plasma contained in a silicone tip, HTA uses heated saline that circulates throughout the uterine cavity for ten minutes. During the treatment, your physician maintains continuous visualization of your uterus, while the saline ensures complete coverage of all endometrial tissue.
While Minerva ES delivers the best clinical results of all endometrial ablation treatments available today1, the Genesys HTA results can be the difference between AUB and a normal, regular period.
For many women, that’s just right.
Treatments for polyps & fibroids
The most advanced device for the safe and fast removal of fibroids (myomectomy) & polyps (polypectomy).
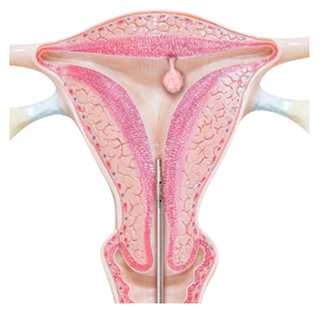
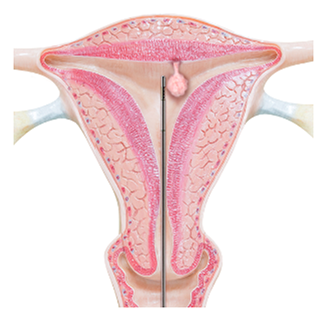
Symphion removes fibroids and polyps through the vagina and cervical canal, without the need for incisions. After slightly dilating the cervical canal, a thin tube with a tiny camera at the end is inserted through the cervix and into the uterus.
A smaller wand-like device is then inserted through the tube and into the uterine cavity. At the tip is a window that enables the removal of fibroids and polyps without using blades.
Tissue from the uterine cavity is continuously removed via the window. When all tissue has been removed, the wand and tube are removed from the uterus.
Symphion uses RF plasma technology and suction to safely remove fibroid tissue from the uterine cavity. This technology eliminates the need for blades, which cut fibroid tissue into small pieces.
Why physicians use Symphion
Minimally invasive
No incisions
No blades
Not a laparoscopic morcellator*
*for more on this download the patient guide below
Treatment for polyps
Modern polyp removal in your doctor’s office
Resectr is a small device that can be used to remove polyps in the exam room at your doctor’s office. This handheld device replaces old-fashioned tools like forceps.
With Resectr, your physician quickly removes polyps without pulling or tugging them out.
Which is nice, right?
Polyps can be just as disruptive to your life as fibroids, and they have the potential to become cancerous.5 So, if you are experiencing symptoms of polyps, ask your gynecologist for a diagnostic hysteroscopy.
You deserve to be heard
A consultation with a gynecologist who understands AUB is what you need and deserve.
CAUTION: Federal law (USA) restricts this device to sale by or on the order of a physician. Rx only. The physician using the system must have sufficient and adequate experience in performing procedures in the uterine cavity, such as IUD insertion or dilation and curettage (D&C), and diagnostic hysteroscopy. Prior to use, please see the complete Instructions for Use for more information on Indications, Contraindications, Warnings, Precautions, Adverse Events, and Operator’s Instructions.
- Laberge P, Garza-Leal J, Fortin C, Grainger D, Johns DA, Adkins RT, Presthus J, Basinski C, Swarup M, Gimpelson R, Leyland N, Thiel J, Harris M, Burnett PE, Ray GF. A Randomized Controlled Multicenter US Food and Drug Administration Trial of the Safety and Efficacy of the Minerva Endometrial Ablation System: One-Year Follow-Up Results. J Minim Invasive Gynecol. 2017 Jan 1;24(1):124-132.
- Minerva Surgical Endometrial Ablation System Operator’s Manual L0001 Rev. E. Available at: https://minervasurgical.com/resources/minerva-es-ifu/ Accessed July 09, 2021.
- NovaSure Advanced Impedance Controlled Endometrial Ablation System. Instructions For Use and Controller Operator’s Manual. Available at: https://www.hologic.com. Accessed July 09, 2021. (Not based on a head-to-head study).
- Genesys HTA. Directions for Use. Available at: https://minervasurgical.com/resources/genesys-hta-directions-for-use/ Accessed July 09, 2021.
- Uterine Polyps. Available at: https://my.clevelandclinic.org/health/diseases/14683-uterine-polyps Accessed July 09, 2021.
Minerva Surgical does not provide medical diagnosis, treatment or engage in the practice of medicine. There are potential risks with any medical procedure. These procedures may not be appropriate for all patients, and all patients may not benefit. For information about risks, visit minervasurgical.com/safety . Rx Only.
Minerva The Uterine Health Company, Minerva ES, Symphion and Genesys HTA are registered trademarks of Minerva Surgical, Inc.
Resectr is a trademark of Minerva Surgical, Inc.
All other trademarks are property of their respective owners.
© Minerva Surgical, Inc. 4255 Burton Drive, Santa Clara, CA 95054. All rights reserved. K0123-04 Rev. B